Technology
Cells
In order to produce an immunocompatible vascular graft, the patient's own endothelial cells are the lining of choice. We use established procedures to isolate and expand endothelial cells from small vascular samples.
For research purposes, like evaluating seeding procedures, endothelial cells isolated from umbilical veins are a cheap and readily available alternative. We cooperate with two OB/GYN departments to obtain umbilical cords as cell sources.
Scaffolds
We evaluate several scaffolds as the basic structure of a vessel graft.
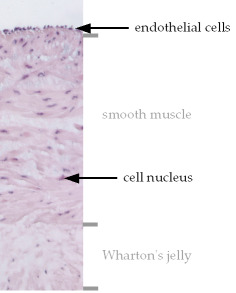
Cross section of an intact human umbilical vein wall
Denuded umbilical veins
We have established procedures to remove the native endothelium from umbilical veins, leaving the smooth muscle cell layer intact and functional. This "living scaffold" serves as an advanced starting point for vessel grafts. We are developing methods to improve cryostorage of umbilical veins.
Decellularized umbilical veins
We have developed a method to remove living cells from umbilical veins, leaving only the extracellular matrix. Although this material is void of any cellular function, it can be stored indefinitely, and there is no risk of rejection.
Microcrystalline Cellulose
Microcrystalline Cellulose (MC) is a bacterial product which can be grown in almost any form. After sterilization and washing, MC consists almost entirely of cellulose and water and can be stored indefinitely. Humans do not possess any enzymes to degrade cellulose. Therefore, MC-based grafts are expected to be stable indefinitely. MC is kindly provided by bioregeneration.
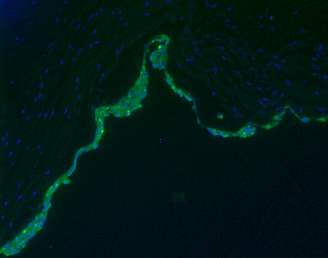
Denuded umbilical vein seeded with fluorescence-labelled endothelial cells
Perfusion Bioreactors
We operate perfusion systems to seed scaffolds and to grow tissue-engineered vessels. These bioreactors were manufactured according to our specifications and serve our needs for evaluating seeding techniques and for short-term experiments.
Advanced Perfusion System
We are developing an advanced perfusion system which adds tissue engineering capabilities to a regulated mock circulation. This system will allow regulation of pressure, flow rates, and shear forces, measurement of compliance, and measurement of substance effects without compromising the integrity of the developing graft.

